Thick film interpretation: Difference between revisions
From MalariaETC
No edit summary |
No edit summary |
||
| (65 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
---- | |||
<span style="font-size:90%">'''Navigation:''' [[MalariaETC Index|Return to Main Malaria Index]]''</span> | |||
---- | |||
</br> | </br> | ||
{| class="wikitable" style="border-style: none; border-width: 2px; border-color: gainsboro; color:black" | {| class="wikitable" style="border-style: none; border-width: 2px; border-color: gainsboro; color:black" | ||
| Line 4: | Line 7: | ||
|} | |} | ||
</br> | </br> | ||
<span style="font-size:90%">A thick film is prepared by placing a small drop of blood on a slide then spreading it in a circular motion. The thick layer acheived is then air-dried without fixation.</br></br>The principles are: | <span style="font-size:90%">A thick film is prepared by placing a small drop of blood on a slide then spreading it in a circular motion. The thick layer acheived is then air-dried without fixation. | ||
<span style="font-size:90%"> | </br></br>'''The principles are:'''</br> | ||
<span style="font-size:90%"> | *<span style="font-size:90%">The blood layer will be many layers thick (varying from 6-20 accross the specimen) | ||
<span style="font-size:90%"> | *<span style="font-size:90%">The erythrocytes are unfixed, so will be lysed during staining appearing only as debris. | ||
<span style="font-size:90%"> | *<span style="font-size:90%">The Giemsa stain will stain and distinguish the remaining white cells and parasites. | ||
</br></ | *<span style="font-size:90%">This concentration effect allows parasites to be detected with high sensitivity | ||
</br> | |||
Typical appearances of a case of ''P.falciparum'' with easily detected trophozoites are shown below.</br></br> | |||
<gallery mode="nolines" heights=200px widths=200px> | |||
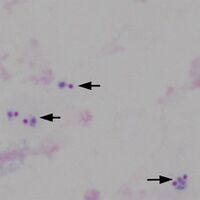
File:TF1b.jpg|<span style="font-size:80%">Low magnification view for scale, 3 regions marked</span>|link={{filepath:TF1b.jpg}} | |||
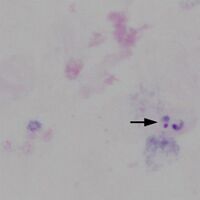
File:TF1c.jpg|<span style="font-size:80%">Region A: a single disrupted trophozoite</span>|link={{filepath:TF1c.jpg}} | |||
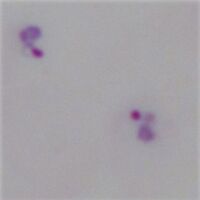
File:TF1d.jpg|<span style="font-size:80%">Region B: 5 trophozoites in three group</span>|link={{filepath:TF1d.jpg}} | |||
File:TF1e.jpg|<span style="font-size:80%">Region C: 2 trophozoites in one group</span>|link={{filepath:TF1e.jpg}}</gallery>" | |||
</br> | |||
<span style="font-size:90%">Note the differences in recognition - the typical ring form and vacuole of the parasite are not as easy to distinguish and chromatin dots may appear to separate from parasite cytoplasm while the absence of intact red cells takes away important clues to parasite size, distribution within the red cell, and any red cell changes. This is illustrated in the image below</span> | |||
<gallery mode="nolines" heights=200px widths=200px> | |||
File:2_Trophs_MP.jpg|<span style="font-size:80%">Two ring frms of ''P.falciparum''</span>|link={{filepath:2_Trophs_MP.jpg}} | |||
File:3_Trophs_HP.jpg|<span style="font-size:80%">High-power image of the parasites</span>|link={{filepath:3_Trophs_HP.jpg}} | |||
</gallery> | |||
'' | </br> | ||
---- | |||
'''Detailed Sections:'''</br></br> | |||
''' | |||
''' | '''Section 1: strengths and weaknesses of thick malaria films''' a comparison different morphological approaches to malaria diagnosis. | ||
</br><span style="font-size:200%">→</span> [[Thick_vs_Thin_film_comparison|Click to see comparison of thick and thin film approaches]]</br></br> | |||
'''Section 2: recognising parasites on thick films''' Initial assessment - recognising staining debris and identifying parasites. | |||
</br><span style="font-size:200%">→</span> [[Thick films - parasites and debris|Click to see the approach to identify parasites and distinguish debris]]</br></br> | |||
'''Section 3: Species identification on thick films''' - the possibilities and limitations of species identification. | |||
</br><span style="font-size:200%">→</span> [[Thick films - parasites identification|Click to see examples of malaria species, stage or pigment on thick films]]</br></br> | |||
---- | |||
Latest revision as of 10:00, 27 February 2025
Navigation: Return to Main Malaria Index
| OVERVIEW OF THICK FILMS |
A thick film is prepared by placing a small drop of blood on a slide then spreading it in a circular motion. The thick layer acheived is then air-dried without fixation.
The principles are:
- The blood layer will be many layers thick (varying from 6-20 accross the specimen)
- The erythrocytes are unfixed, so will be lysed during staining appearing only as debris.
- The Giemsa stain will stain and distinguish the remaining white cells and parasites.
- This concentration effect allows parasites to be detected with high sensitivity
Typical appearances of a case of P.falciparum with easily detected trophozoites are shown below.
-
Low magnification view for scale, 3 regions marked
-
Region A: a single disrupted trophozoite
-
Region B: 5 trophozoites in three group
-
Region C: 2 trophozoites in one group
"
Note the differences in recognition - the typical ring form and vacuole of the parasite are not as easy to distinguish and chromatin dots may appear to separate from parasite cytoplasm while the absence of intact red cells takes away important clues to parasite size, distribution within the red cell, and any red cell changes. This is illustrated in the image below
-
Two ring frms of P.falciparum
-
High-power image of the parasites
Detailed Sections:
Section 1: strengths and weaknesses of thick malaria films a comparison different morphological approaches to malaria diagnosis.
→ Click to see comparison of thick and thin film approaches
Section 2: recognising parasites on thick films Initial assessment - recognising staining debris and identifying parasites.
→ Click to see the approach to identify parasites and distinguish debris
Section 3: Species identification on thick films - the possibilities and limitations of species identification.
→ Click to see examples of malaria species, stage or pigment on thick films