Thick film interpretation: Difference between revisions
From MalariaETC
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
|} | |} | ||
</br> | </br> | ||
<span style="font-size:90%">A thick film is prepared by placing a small drop of blood on a slide then spreading it in a circular motion. The thick layer acheived is then air-dried without fixation.</br> | |||
IMAGE | IMAGE | ||
</br>The principles are:</br> | |||
*<span style="font-size:90%">The blood will therefore be many layers thick (around 6-20) compared with the single layer of a thin film | *<span style="font-size:90%">The blood will therefore be many layers thick (around 6-20) compared with the single layer of a thin film | ||
*<span style="font-size:90%">The erythrocytes are unfixed so will be lysed during staining appearning only as debris. | *<span style="font-size:90%">The erythrocytes are unfixed so will be lysed during staining appearning only as debris. | ||
| Line 12: | Line 12: | ||
*<span style="font-size:90%">This allows parasites to be detected with high sensitivity using fewer microscopic fields | *<span style="font-size:90%">This allows parasites to be detected with high sensitivity using fewer microscopic fields | ||
</br> | </br> | ||
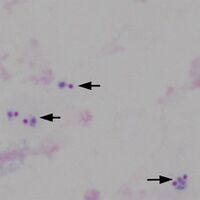
Typical appearances of a case of ''P.falciparum'' with easily detected trophozoites are shown below.</br></br> | |||
<gallery mode="nolines" heights=200px widths=200px> | <gallery mode="nolines" heights=200px widths=200px> | ||
File:TF1b.jpg|<span style="font-size:80%">Low magnification view for scale, 3 regions marked</span>|link={{filepath:TF1b.jpg}} | File:TF1b.jpg|<span style="font-size:80%">Low magnification view for scale, 3 regions marked</span>|link={{filepath:TF1b.jpg}} | ||
| Line 21: | Line 21: | ||
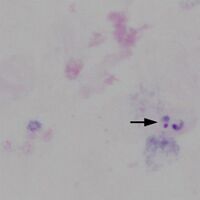
<span style="font-size:90%">Note the differences in recognition - the typical ring form and vacuole of the parasite are not as easy to distinguish and chromatin dots may appear to separate from parasite cytoplasm while the absence of intact red cells takes away important clues to parasite size and distribution.</span> | <span style="font-size:90%">Note the differences in recognition - the typical ring form and vacuole of the parasite are not as easy to distinguish and chromatin dots may appear to separate from parasite cytoplasm while the absence of intact red cells takes away important clues to parasite size and distribution.</span> | ||
IMAGE | IMAGE AT HIGH POWER | ||
<span style="font-size:90%">Some strengths and weaknesses of each approach are summarised in the table below:</br></span> | <span style="font-size:90%">Some strengths and weaknesses of each approach are summarised in the table below:</br></span> | ||
Revision as of 10:49, 11 February 2025
| OVERVIEW OF THICK FILMS |
A thick film is prepared by placing a small drop of blood on a slide then spreading it in a circular motion. The thick layer acheived is then air-dried without fixation.
IMAGE
The principles are:
- The blood will therefore be many layers thick (around 6-20) compared with the single layer of a thin film
- The erythrocytes are unfixed so will be lysed during staining appearning only as debris.
- The Giemsa stain will therefore stain and distinguish the remaining white cells, parasites.
- This allows parasites to be detected with high sensitivity using fewer microscopic fields
Typical appearances of a case of P.falciparum with easily detected trophozoites are shown below.
-
Low magnification view for scale, 3 regions marked
-
Region A: a single disrupted trophozoite
-
Region B: 5 trophozoites in three group
-
Region C: 2 trophozoites in one group
"
Note the differences in recognition - the typical ring form and vacuole of the parasite are not as easy to distinguish and chromatin dots may appear to separate from parasite cytoplasm while the absence of intact red cells takes away important clues to parasite size and distribution.
IMAGE AT HIGH POWER
Some strengths and weaknesses of each approach are summarised in the table below:
| COMPARISON OF THICK AND THIN FILMS | ||
|---|---|---|
| Feature | Thick Film | Thin Film |
| Sensitivity for detection | Higher: detects low parasitaemia ~5–10 parasites/µL | Lower: generally needs ~50 parasites/µL for reliable detection) |
| Species Identification | Poor: RBC morphology lost and species-specific features may be difficult | Excellent: Parasite morphology and RBC characteristics are readily observed |
| Quantification of parasitaemia | Difficult: requires estimation so is imprecise | Easier: parasites can be counted per number of RBCs |
| Preparation and staining | Longer: requires air drying before careful staining to avoid artefact | Faster: films are fixed and stained immediately with clearer morphology |